If there isn’t any number or signs, then it means that atom has no charge and is neutral. Rules to Finding Number of Protons, Neutrons, and Electrons # of protons = atomic number # of neutrons = mass number – atomic number. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and electrons in an atom or in an ion. It also explains the differe. The number of protons in an atom is not changeable so you can add or subtract electrons to get the charge. Example: Zn2+ (meaning the ion has exceeded 2 protons over the number of electrons.) 30 (Atomic no. Of Zinc) — 2 = 28 electrons. Finding the number of Neutrons. Electrons fill in shell and subshell levels in a semiregular process, as indicated by the arrows above. After filling the first shell level (with just an s subshell), electrons move into the second-level s subshell and then into the p subshell before starting on another shell level. Because of its lower energy state, the 4s orbital fills before the 3d, and later s orbitals fill similarly (for.
Lewis structure of NO2 (Nitrogen dioxide) is drawn in this tutorial step by step. You can learn basics of how to draw a lewis structure properly from this example.
This is a special case of lewis structure drawing because, there is a unpaired electron on nitrogen atom. You will see this case when we draw the lewis structure of NO2 in next sections of this tutorial.
Steps of drawing NO2 lewis structure
Following steps are required to draw the NO2 lewis structure and they are explained in detail in this tutorial.
- Find total number of electrons of the valance shells of nitrogen and oxygen atoms
- Total electrons pairs
- Center atom selection
- Put lone pairs on atoms
- Check the stability and minimize charges on atoms by converting lone pairs to bonds.
Drawing correct lewis structure is important to draw resonance structures.
Total number of electrons of the valance shells of nitrogen and oxygen atoms
Nitrogen and oxygen are located at VA and VIA groups respectively in the periodic table. So nitrogen has five electrons in its valence shell. In oxygen atom, there are six electrons in its valence shell.
- Total valence electrons given by nitrogen atom = 5
There are two oxygen atoms in NO2, Therefore
- Total valence electrons given by oxygen atoms = 6 *2 = 12
- Total valence electrons = 5 + 12 = 17
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, NO2, Total pairs of electrons are 8 and one electron exists as unpaired electron because 17 cannot be divided exactly by 2.

Center atom of NO2
To be the center atom, ability of having greater valance is important. Therefore nitrogen has the more chance to be the center atom (See the figure). So, now we can build a sketch of NO2.
Lone pairs on atoms
There are already two N-O bonds in the sketch. Therefore only six valence electrons pairs are remaining.
Mark those six valence electrons pairs as lone pairs in outside atoms (on oxygen atoms). One oxygen atom will take three lone pairs following the octal rule (oxygen and nitrogen atoms cannot keep more than eight electrons in their valence shells).
Mark unpaired electron on nitrogen atom.
Check the stability and minimize charges on atoms by converting lone pairs to bonds
The drawn structure is not a stable one because both oxygen atoms and nitrogen atoms have charges. Also nitrogen has a +2 charge and it decreases the stability of that structure.
Now, we should try to minimize charges by converting lone pair(s) which exist on oxygen atoms to bonds. So convert one lone pair of one oxygen atom to a N-H bond.
Now there is a double bond between nitrogen and one oxygen atom. There is a single bond also with nitrogen atom and other oxygen atom.
In new structure, charges of atoms reduced than previous structure. Now there is no any charge on one oxygen atom. Charge of nitrogen atom is also decreased to +1 from +2. Now you understand this structure of NO2 is more stable than previous structure. So, this structure has more chance to be the lewis structure of NO2.
We cannot convert more lone pairs of other oxygen atom to make a bond with nitrogen atom because a nitrogen atom cannot keep more than eight electrons in its valence shell.
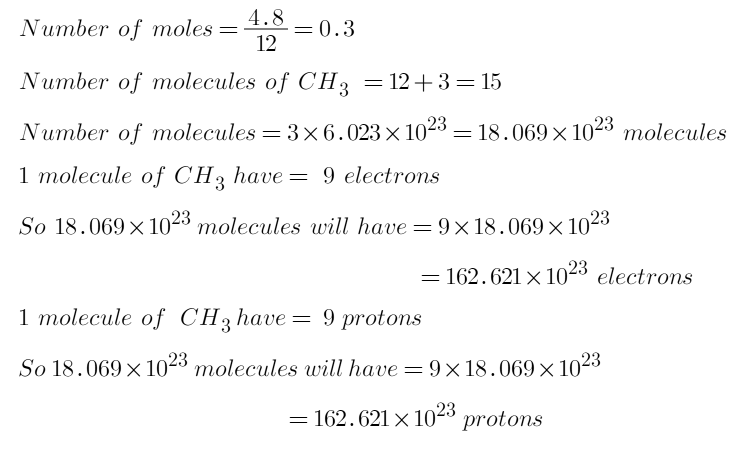
NO2 structure
The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. That means both bonds exist between oxygen and nitrogen are same.
Due to exist of unpaired electron, NO2 is a free radical.
There is a unpaired electron on nitrogen atom in NO2 lewis structure. Cannot it be shifted to an oxygen atom?
In above lewis structure, octal of oxygen atoms are already completed. There is no chance to have more than eight electrons in last shell.
Effect of unpaired electron
NO2 is a free radical. When it is in the presence of sun light, reacts with oxygen gas and produce NO and atomic oxygen. This is an initial step of photochemical smog which is occurred at cities which have high traffics.
Oxidation number of NO2
Oxygen is more electronegative than nitrogen. Therefore electrons of bonds are attracted towards oxygen atoms more than nitrogen.
There are three bonds between (two σ + one π) nitrogen atom and oxygen atoms. De to those bonds, nitrogen partially loses its three electron. So due to that case, nitrogen gets +3 oxidation state. But, wait. When your consider last shell of nitrogen atom, nitrogen belongs only four electrons. So nitrogen atom is lacking one electron and it makes +1 oxidation number. At finally, overall oxidation number of nitrogen is +4 (+3 + 1).
Questions
Ask your chemistry questions and find the answersnumber of π bond pairs in no2
There is only one π bond in NO2 lewis structure.
What is the colour of NO2? I have heard that it is brown colour. Is it true. Why NO2 has a colour?
At room temperature, nitrogen dioxide is a brown colour gas. In lewis structure of NO2, there is a unpaired electron on nitrogen atom. That unpaired electron is the reason for showing colours by NO2 molecule.
NO2 structure?
There is an unpaired electron on nitrogen atom. Therefore the molecule has three electron pairs around nitrogen atom and is trigonal planar for electron pair geometry. The one lone electron exerts a less repulsion than normal on the two bonding oxygen atoms so they are able to spread out more to a 1340 bond angle from the ideal of 1200.
What is the difference of lewis structures of NO2 and NO2-
In the lewis structure of NO2, there is a unpaired electrons on nitrogen atom. But in the NO2- lewis structure, there is lone pair on nitrogen atom.
No Of Electrons In Oxygen
Related lewis structures
P2O5 lewis structureOH- lewis structureAmmonium ion (NH4+) lewis structureH2CO3 lewis structureCategory: Physics Published: March 17, 2016
The answer to this question depends on the situation. We can roughly classify all electrical systems into two categories: static electricity systems and circuit electricity systems. Note that all electrical effects are actually part of one unified set of physical laws. This classification is therefore ultimately arbitrary and over-simplified. However, this classification is sufficient for our current purpose of understanding electric current.
A static electricity system involves the flow of electric current as a result of a buildup of electric charge somewhere. Such a system does not involve a closed electrical circuit. Examples of this type of system include lightning and the sparks you get when you rub your feet on a carpet. Electrons naturally repel each other. When a lot of electrons get piled up in one place, they can push on each other so strongly that some of the electrons get pushed right off of the object. They end up getting pushed out through the air, the water, or whatever surrounds the object. We call a collection of moving electrons an electric current, therefore a buildup of charge can drive a current. The electrons simply flow away from the pile and ultimately end up attached to atoms in the environment. In this way, we can have an electric current even if we don't have a complete electrical circuit. In air, an electrical current takes the form of dark discharge, corona discharge, or sparks (depending on if the current is weak, medium strength, or strong, respectively). Note that the name 'static electricity' is a poor name since the electric charge is not always stationary in this type of system. More accurate names would be 'non-circuit electricity' or 'charge buildup electricity.'
Since charge buildup is the cause of the electric current in static electricity systems, the current will stop flowing once the buildup goes away. As the electrons flow away from the pile, the pile gets smaller. Eventually, the pile of excess electrons is gone (the electrons that are needed to keep the molecules neutral still remain, but they don't do much). Quite literally, electricity stops flowing because the source runs out of excess electrons. This is why lightning bolts and the sparks between statically-charged socks go away quickly. It's not that electrons are destroyed. Rather, they are leaked away to distant points until none remain.
In contrast, circuit electricity systems involve the flow of electric current through a closed loop. This current is the result of a charge pump operating somewhere in the loop. This pump is also called a voltage source and can take the form of a battery, a solar cell, a generator, or the cord from a power grid. The pump creates a voltage difference along the circuit which drives charges like electrons through the circuit. The pump can either constantly pump electrons in one direction, which leads to a direct current (DC), or it can periodically switch off the direction in which it is pumping electrons, which leads to an alternating current (AC). For simplicity, let's focus on direct current.
As the electrons flow through the circuit, they flow down the potential energy slope that is created by the voltage. Once they reach the pump at the end of the circuit, the low-energy electrons are boosted back up to a high potential energy so that they can start flowing through the circuit again. The situation is a bit like an artificial waterfall in your backyard. Water flows down the waterfall and into a pool because of the natural pull of gravity, just like how electrons flow through the circuit because of the pull of the applied voltage. A water pump then pushes the water in the pool back up to a high energy state at the top of the waterfall, just like how a battery pushes electrons back up to a higher energy state at the beginning of the circuit. The cycle then repeats.
Since the pumping of charge is the cause of the electric current in a circuit electricity system, the current will never stop flowing as long as the pump remains on and the circuit remains uninterrupted. Circuits don't create, destroy, use up, or lose electrons. They just carry the electrons around in circles. For this reason, circuit electrical systems can't really run out of electrons. The energy delivered through a circuit is not the result of electrons existing in the circuit. Electrons always exist in the circuit as part of the atoms and molecules that make up the circuit. The electrical energy that is delivered is the result of the electrons moving through the circuit. Turn off the pump (i.e. disconnect the battery), and the electrons stop moving through the circuit. But the electrons don't go away. They are still there as a natural part of the materials in the circuit.
No Of Electrons In Boron
As I said before, the categorization of systems into static and circuit systems is somewhat arbitrary and oversimplified. Real electrical systems contain a combination of both effects. For instance, a circuit often contains a capacitor. While the circuit acts overall like a circuit electricity system, the capacitor acts more like a static electricity system. As a result, a capacitor can indeed run out of electrons. As soon as one side of the capacitor is depleted of electrons, the electric current stops flowing through the capacitor. At that moment, the part of the circuit containing the capacitor switches from acting like a circuit electricity system to a static electricity system. This happens in the sense that current is now being stopped by a lack of electrons, and not by the lack of an electron pump or the lack of a complete circuit.
